Abstract
Introduction:Acute myeloid leukemia (AML) is heterogenous disease with a range of cytogenetic and molecular changes. Several molecular mutations identified in AML patients at diagnosis have prognostic implications and play important roles in guiding induction and consolidative treatment decisions. The prognostic impact of mutations peri allogeneic stem cell transplant are less well characterized. In this study, we examine the significance of pre and by D100 Post-transplant mutation status in AML patients underwent Fludarabine/Melphalan conditioned reduced intensity allogeneic stem cell transplant (SCT).
Methods:AML patients who are in morphologic complete remission (CR1 or greater) with available molecular mutation at diagnosis, within 6 weeks prior to allogeneic SCT, and by 100 days post-transplant were included. Variables analyzed included baseline demographics, clinical variables (CIBMTR disease risk index (DRI), type of transplant, ELN risk, performance status) and 23 recurring molecular mutations. Analysis was also performed by grouping mutations into six pre-defined gene groups based on gene function (Table 2). Multivariable cox regression analysis was adjusted for age, gender, DRI and molecular mutation. Backward selection method was used to select the best combination of genes that is associated with overall survival (OS) and relapse-free survival (RFS).
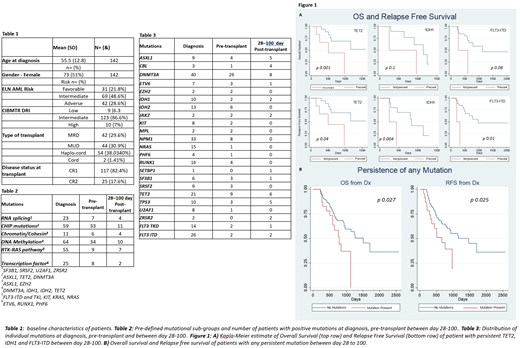
Results: A total of 142 AML patients with molecular genetic data available from 2014 to June, 2020 at Weill Cornell Medicine/New York Presbyterian Hospital were analyzed. Clinical characteristics of the patients are summarized in Table 1. The median age was 58 years (range 20 -78). Total of 261 mutations were detectable at diagnosis (Table 3). Prior to allo SCT and by D100, the detectable mutations were 87 and 40 respectively, which represent 56 and 26 patients. High-dose chemotherapy was less effective on clearing DNMT3A, ASXL1, TET2 (DAT) or IDH mutations, resulting in over-representation of DAT and IDH mutations prior to transplant.
With a median follow-up time of 25 months, the median overall survival for the group was 40.8 months. The presence of mutations in TP53 at diagnosis was associated with worse OS by both univariate (HR 3.67, p=0.0030, CI 1.56-8.68) and multivariate analysis (HR 4.75, p=0.0014, CI 1.82-12.39) with median OS reduced from 49.3 to 19.3 months (p=0.002). High CIBMTR DRI (HR 0.17, p=0.0018, CI 0.05-0.51) predicted reduced OS and RFS, and Age >60 at diagnosis was associated with worse OS (HR 1.7 CI 1.04-3, p 0.03).
Presence of any molecular mutation prior to transplant did not impact OS or RFS. For patients with any persistent mutations by D100 post-transplant, both OS (HR 2.04, p 0.027, CI 1.08-3.8) and RFS (HR 1.99, p 0.025, CI 1.09-3.6,) were reduced in the univariate analysis, but not on multivariate analysis (HR 1.88, p 0.5, CI 0.99-3.49). Analysis based on six mutational groups (table 2) did not show any difference in their OS or RFS. However, worse RFS was independently associated with persistent IDH1 (HR 3.8, p 0.004, CI 1.07-56,), TET2 (HR 3.9, P 0.04, CI 1.04-14.1), and FLT3-ITD (HR 4.5, p 0.01, CI 1.7-52). Worse OS was independently associated with persistent TET2 (HR 3.9, p 0.013, CI 1.04-14.1), with a trend towards worse OS for IDH1, FLT3-ITD, with a trend towards worsening OS and RFS for ASXL1 (OS HR 7.4, p 0.06, CI 0.86 -63; RFS HR 4.9, p 0.06, CI 0.9-26) and DNMT3A (OS HR 2.3, p 0.12, CI 0.86-6.9; RFS 2.9, p 0.08, CI 0.98-8). Association with worse clinical outcomes remained significant after multivariate analysis for TET2 (both OS HR 3.98 p 0.041, CI1.07- 32 and RFS HR 5.8, p 0.032, CI 1.1- 29), IDH1 (RFS HR 8.02, p 0.049, CI 1.02 - 65) and FLT3-ITD (RFS HR 11.4, p0.010, CI 2.2- 80).
Conclusions:Presence of TP53 mutations was associated with worse OS. Presence of pre-transplant mutation did not impact RFS or OS. Persistent presence of mutations in TET2, IDH1 and FLT3-ITD after Fludarabine/melphalan conditioning regimen allogeneic SCT were associated with shorter RFS and OS (in the case of TET2) independent of CIBMTR DRI. This analysis supports association of adverse outcomes in AML patients with selected persistent mutations by D100 post-transplant in reduced intensity transplant setting. Post-transplant strategies that can further eliminate persistent mutations should be investigated in prospective studies.
Lee: Pin Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Innate: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees. Desai: Kura Oncology: Consultancy; Bristol Myers Squibb: Consultancy; Agios: Consultancy; Takeda: Consultancy; Janssen R&D: Research Funding; Astex: Research Funding. Ritchie: Protaganist: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Speakers Bureau; Celgene/BMS: Consultancy, Other: travel support, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding; ARIAD Pharmaceuticals: Ended employment in the past 24 months, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria; Astellas: Consultancy, Research Funding; NS Pharma: Research Funding; Abbvie: Consultancy, Honoraria; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Roboz: MEI Pharma - IDMC Chair: Consultancy; Daiichi Sankyo: Consultancy; Helsinn: Consultancy; Jazz: Consultancy; Bristol Myers Squibb: Consultancy; Glaxo SmithKline: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Otsuka: Consultancy; Celgene: Consultancy; Mesoblast: Consultancy; Blueprint Medicines: Consultancy; Jasper Therapeutics: Consultancy; AbbVie: Consultancy; Actinium: Consultancy; Agios: Consultancy; Amgen: Consultancy; Astex: Consultancy; Astellas: Consultancy; AstraZeneca: Consultancy; Bayer: Consultancy; Janssen: Research Funding; Pfizer: Consultancy; Roche/Genentech: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal